
The India-UK FTA is expected to promote the free movement of goods and services, significantly impacting access to pharmaceuticals, medicines, and devices. It is crucial for health activists and policymakers to continuously monitor and analyse the FTA’s impact on the population’s health and well-being, making necessary adjustments as needed. Although the Conservative government of the UK negotiated the Free Trade Agreement, the newly elected Labour Party has made public its commitment to finalise it.
Many nations negotiate and finalise new FTAs bilaterally, promoting significant economic benefits by removing or lowering trade barriers for products and services. Nevertheless, bilateral and regional FTAs are often viewed as advancing the trade liberalisation agenda, given the prolonged Doha Round of multilateral negotiations. However, FTAs may need to ensure public health exceptions and guarantees.
READ | Can Kerala balance social spending and fiscal goals
There is a strong indication that the FTA favours the UK’s trade interests. According to the UK Department of International Trade, an FTA between the UK and India allows British and Indian businesses to benefit from lower trade costs, boosts economic activity in both countries, and provides opportunities for increased specialisation. Businesses across both countries could gain access to cheaper inputs, benefiting existing supply chains and encouraging new ones. An agreement could benefit consumers directly through increased choice, better product quality, and lower prices.
In May 2021, Prime Ministers Modi and Johnson committed to an Enhanced Trade Partnership, which could double trade by 2030, strengthening the relationship and invigorating both economies through an FTA. This Enhanced Trade Partnership is part of a more comprehensive 2030 Roadmap covering the full spectrum of the UK-India bilateral relationship.
By removing tariffs and non-tariff measures, the India-UK FTA could significantly enhance the competitiveness of UK businesses, opening up new opportunities for increasing UK exports to India and fostering optimism among policymakers and trade analysts.
Public health impact of India-UK FTA
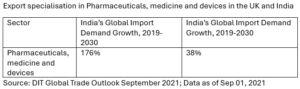
According to the UK Department for International Trade (2022), the UK-India FTA presents a significant opportunity for the UK pharmaceutical sector. India’s increasing demand for chemicals, including pharmaceutical products, has made it the fifth-largest importer globally, valued at £47 billion in 2019. This growth trend provides a hopeful outlook for the pharmaceutical industry’s future under the FTA.
Despite India’s tariffs peaking at 100%, the potential for increased UK exports to India under the FTA is encouraging. The UK’s exports to India in the chemicals and pharmaceuticals sector have increased by 60% since 2010, reaching £43 million in 2019. This growth trajectory should encourage stakeholders in the pharmaceutical industry.
All UK sectors are expected to experience sustained expansion following the implementation of the India-UK FTA, which is actively negotiated between India and the United Kingdom and is expected to bolster bilateral trade, valued at approximately GBP 38.1 billion annually. This projection should instilconfidence and reassurance among government officials.
The India-UK FTA is a landmark deal for India, marking its first comprehensive agreement with an industrialised nation. This agreement is expected to significantly boost India’s trade, potentially surpassing the current $20 billion mark. This information should make health activists and policymakers more aware of the potential impact of the FTA.
The agreement explicitly states the UK government’s commitment that the National Health Service (NHS), its services, and the cost of medicines are not on the table. The FTA will not accept any provisions that would increase drug costs for the NHS. Protecting the NHS is a fundamental principle of the FTA trade policy, and the UK is committed to this during negotiations with India.
The UK government has reaffirmed its commitment to international labour standards and assured that parties will not waive or fail to enforce their domestic labour protections in ways that create an artificial competitive advantage. These measures allow the UK to protect its regulatory sovereignty, maintain its integrity, and provide meaningful protection for labour rights. The FTA also provides mechanisms for implementing, monitoring, and resolving disputes over labour provisions. However, the impact on the Indian labour force is not clear.
The trade agreement also recommits to gender equality. UK trade negotiators will promote women’s access to the full benefits and opportunities of this agreement as workers, business owners, entrepreneurs, and consumers. They will seek cooperation to address barriers that disproportionately affect women in trade. The importance of upholding protections for women’s gender equality in the UK is recognised, but no such guarantees are explicit in the FTA documents regarding Indian women’s equity in trade.
On May 25th, the Department for International Trade launched a consultation to seek input from consumers and businesses across all sectors. The online consultation questionnaire had 94 questions. All respondents were asked the same core 24 questions and seven questions for identification and data protection purposes. Additionally, demographic and logistical questions were targeted at each group. Individuals and NGOs were asked ten questions, public sector bodies five questions, businesses 24 questions, and business associations 14 questions. In total, 283 responses were received.
Based on the consultation, 18 policy areas were identified, and responses were presented. These policy areas include tariffs, rules of origin, customs procedures, services, digital, product standards, regulation and certification, sanitary and phytosanitary (SPS) measures, competition, state-owned enterprises and subsidies, government procurement, and intellectual property. Other areas include investment, innovation, environment and climate change, trade remedies, dispute settlement, SME policy, labour standards, and gender equality and women’s economic empowerment. However, the health and well-being of the Indian population were not reflected in the policy areas discussed as part of the FTA preparation. The potential impact of the FTA on access to medicine, pharmaceuticals, and devices needs careful consideration.
Flexibilities are essential to the TRIPS or FTA agreement. A monitoring mechanism should ensure the FTA’s commitment to the Doha Declaration on the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement and Public Health and agreed-upon flexibilities that support access to medicines, particularly during public health emergencies in developing countries. Strengthening civil society action to protect the health and well-being of the population in the context of the FTA is necessary.
FTAs pose a challenge for health activists and policymakers, necessitating appropriate actions to monitor and advocate for change. This is particularly important for public health advocates concerned with the broader commercial determinants of health. Identifying strategies that advance health considerations in trade policymaking addresses the immediate FTA challenge and provides valuable insights for promoting health in other policy areas.
On November 2nd, 2022, the ‘Missing Medicines Coalition’ sent a letter to Kemi Badenoch, Secretary of State for International Trade, expressing concern over a leaked intellectual property (IP) chapter believed to be the UK’s opening proposal for the UK-India FTA. They requested that the UK government urgently rethink its approach and open negotiations to full public transparency and parliamentary scrutiny, including meaningful engagement on intellectual property provisions.
The letter acknowledged that India’s longstanding ability to produce quality-assured, affordable medicines for diseases like HIV, TB, viral hepatitis, malaria, and others relies upon its carefully drafted intellectual property laws and medical regulatory processes, which balance the monopoly rights of manufacturers with everyone is right to health. India also supplies the NHS with 25% of all medicines used in the UK. Any action that curtails India’s ability to produce quality, cost-effective medicines threatens the financial sustainability of the NHS and puts patients’ lives at risk.
One persistent barrier to health equity is the lack of intersectoral action for health and health equity in all areas of policymaking. This need is particularly acute in trade policymaking, where government economic interests often precede health goals in promoting export markets.
While there is extensive knowledge about the impacts of economic determinants on health, there is a significant gap in understanding how to advance health goals in policymaking in areas not traditionally part of the health portfolio. This gap underscores the need for further research and development in this area.
Trade policy intersects with public health in critical areas, such as access to medicines, nutrition, tobacco control, and asbestos harm. Including access to knowledge as a social determinant of health, influenced by copyright and intellectual property rules, is particularly noteworthy.
According to Townsend and colleagues (2021), while domestic concerns about protecting regulatory space for access to generic medicines and tobacco control emerged, other health issues like alcohol control and nutrition and food systems did not receive attention. Their analysis suggests sixteen key factors that shaped attention to these different health issues, including the strength of exporter interests, the extent of political will of Trade and Health Ministers, framing of health issues, support within major political parties, exogenous influencing events, public support, the strength of available evidence, and the presence of existing domestic legislation and international treaties.
Various strategies are needed to advance health goals in trade policymaking, including gaining positive media coverage, developing leadership positions taken by Trade and Health Ministers to support health, mobilising public opinion, and gaining political party support. These circumstances were frequently linked; for instance, good media coverage was strongly related to rising public opinion and political party support for a health issue.
Public health advocates (including members of civil society, academia, and government officials) employ various strategies to impact media coverage, create public sentiment, generate political party endorsement, and provide ministerial leadership. These include joining forces with others, influencing policy actors through strategic frameworks, taking advantage of exogenous occurrences, employing legal tactics, and moving forums to locations more supportive of health objectives.
Health impact assessments (HIA) have provided valuable technical evidence to promote access to medicines in trade agreements. Public health advocates must constantly debunk industry claims with influential evidence, including personal stories and economic evidence, to support evidence-informed debate.
Public health advocates must work in alliances with civil society organisations and larger coalitions, including government representatives, members of civil society, and other organisations and specialists who can provide support. By uniting as a coalition, public health advocates can counter the influence of wealthy, resource-rich nations in trade negotiations. Unofficial alliances involving industry associations, civil society participants, and government health authorities have also been found to have significant sway.
Another strategy is the strategic framing of issues. Policy actors use frames to focus on a particular problem and persuade others of its importance. Access to medicines was identified as a robust framing and norm which shifted debates on intellectual property and trade from private goods to public health.
Human rights framing has also been successfully used, shaping public support and media uptake of pro-health framing in trade debates. Advocates also leveraged exogenous events, seizing windows of opportunity to draw attention to health in trade processes. Exogenous factors outside the trade process included the rise of HIV as a health and security crisis, food pricing and harvest levels, and economic crises.
Public health advocates often use legal arguments in national and international contexts to advance health. For example, the World Health Organisation’s Framework Convention on Tobacco Control (a non-trade treaty) has been used to defend tobacco control measures. However, public health advocates must often manoeuvre across different institutional forums to exert pressure on trade-related policy domains. Various political strategies are needed to advance health when working with government portfolios and departments involved in trade policymaking.
FTAs pose new challenges for public health activists in India, necessitating appropriate actions to monitor and advocate for change. This is particularly important for public health advocates concerned with the broader commercial determinants of health.
Specific monitoring provisions should be included to ensure that the FTA deal does not compromise the health and well-being of the Indian people.
The FTA must have a dedicated public health chapter and policy response to facilitate cooperation between the UK and India on mutual-interest public health issues. The FTA must also provide online information resources to help the public health community ensure that it does not violate the health and well-being interests of the Indian people. Signposts to provisions in other chapters of the agreement that protect and enhance health and well-being should also be provided. The proposed Strategic Trade Advisory Group’s mandate should include regular monitoring reports on the impact of the India-UK FTA on public health.
Dr Joe Thomas is Global Public Health Chair at Sustainable Policy Solutions Foundation, a policy think tank based in New Delhi. He is also Professor of Public Health at Institute of Health and Management, Victoria, Australia. Opinions expressed in this article are personal.

